 There has been increasing interest of late in very slow electroencephalographic (EEG) activity. While this sort of activity has been recorded and studied for many years, and used in biofeedback protocols in several applications, it is only recently gaining popularity as another tool with potential mainstream application to clinical neurofeedback. Whatever the tool, before embarking on a new clinical path, a general understanding of the existing research, as well as the technical and neuro-physiological basics is crucial to a successful experience. While there is no need to get overly carried away by the technical issues, a little knowledge can help avoid misunderstandings and common pitfalls, while hopefully leading to better clinical outcomes.
There has been increasing interest of late in very slow electroencephalographic (EEG) activity. While this sort of activity has been recorded and studied for many years, and used in biofeedback protocols in several applications, it is only recently gaining popularity as another tool with potential mainstream application to clinical neurofeedback. Whatever the tool, before embarking on a new clinical path, a general understanding of the existing research, as well as the technical and neuro-physiological basics is crucial to a successful experience. While there is no need to get overly carried away by the technical issues, a little knowledge can help avoid misunderstandings and common pitfalls, while hopefully leading to better clinical outcomes.
What is DC-EEG?
DC-EEG is a bit of a misnomer, as DC, or direct current, refers to a signal value that is not changing. It is commonly understood to be the baseline about which the oscillating EEG activity varies. Technically, the DC component is the average of the signal, and it happens to turn up as the first term of the Fourier series, or the first value in the frequency domain obtained using the fast Fourier transform (FFT). OK, enough math for now, all this means is that when a very slowly changing EEG signal is described, the term DC is adopted when referring to the signal as DC-EEG.
Most often, the term DC applied to the EEG signal is borrowed from the term DC used to characterize the amplifier being used in the recording. A true DC amplifier does not omit any low frequencies and the DC component of the signal is captured along with all the rest. In most applications, the DC component is selectively omitted from the signal acquisition (the motivations for this will be explained later), and the baseline of the recorded EEG is zero, i.e. the EEG signal oscillates about the zero line. In addition to the DC component, very low frequencies are also omitted, as the truncation can never be instantaneous. What is actually left out of the recording is a range of low frequencies starting at the DC point (0 Hz) and ending at the low cutoff frequency of the system (often around 1-2 Hz).
This brings only benefits as long as the signal of interest is within the frequency range that remains above the cutoff point. When this signal lives below the cutoff frequency, removal of the DC component and the range of frequencies slightly above it will also remove the required signal. This signal of interest, as it applies to the research discussed in this article, refers to EEG activity below 1 Hz, below 0.1 Hz, even below 0.01 Hz – in other words very slowly oscillating neurocortical activity.
Terminology tends to get tangled, but if semantics are respected and time and frequency are considered to be intimately related, it becomes clear that DC-EEG, low frequency EEG and slow cortical potentials (SCP) in fact all refer to the same thing. (Consider: a DC amplifier passes low frequencies, which can be described as slow activity, and cortical potentials are the source of surface EEG). In the field of neurofeedback to date, the different terms tend to describe the particular methodology, as low frequency training and self-regulation of slow cortical potentials may imply different training strategies applied to the same EEG signal.
In the text that follows, when referring to the concept of recording EEG with a DC amplifier, the broad term DC-EEG will be used. When referring to the EEG itself, the terms low frequency EEG and slow cortical potentials will be used according to the context.
Why DC-EEG?
DC-EEG is considered to reflect the general state of neurons and to contribute to the explanation of the mechanisms of surface EEG (Speckmann and Elger, 2005). The origin is reported to be linked to several phenomena, at the same time neuronal, glial and non-neuronal in nature. The SCP is an indicator of relative whole brain state, as slow shifts tend to reflect general activation and inhibition.
Negative shifts of SCPs reflect widespread depolarization of apical dendrites of pyramidal neurons (Birbaumer et al., 1990) and decrease of thresholds for paroxysmal activity. Positive shifts of SCPs are thought to result from inhibitory sources. Dr. Ute Strehl states it this way: “Negative SCPs increase firing probabilities, whereas positive SCPs [inhibit] the respective cell assembly. These neurophysiological considerations suggest an important role for SCPs in the modulation of excitation thresholds of cortical pyramidal cells [the source of surface EEG]” (Langley, 2001). Hinterberger (2004) summarizes as follows: “negativity represents the mobilization or readiness, positivity represents ongoing cognitive and neural performance or inhibition of neuronal activity.”
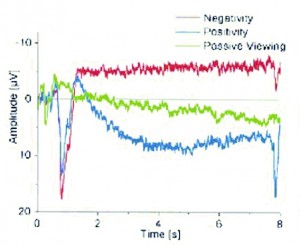
Figure 1. Average EEG waves during voluntary production of cortical negativity (red) and positivity (blue), with passive viewing (no SCP shift) shown in green. Taken from Birbaumer et al., 2003
The early work by Niels Birbaumer and his group at the University of Tubingen, in Tubingen, Germany, showed that control of these SCP shifts can be learned (Kubler et al., 1999; Birbaumer et al., 2000; Kubler et al., 2001, Wolpaw et al., 2002) (Figure 1). This was recently correlated to changes in fMRI data to further validate the previous findings (Birbaumer et al., 2003). Using biofeedback, patients were taught to control their SCPs to produce the positive and negative shifts required to select letters or words in a computer program. This was quite an inspiring undertaking with touching results, as one of the first successful messages typed using the device was a heartfelt thanks to Dr. Birbaumer and his team (Geary, 2002).
Applied to epilepsy, it is believed that suppressing negative SCP shifts can help to limit the electrocortical activation of the brain and control the incidence of seizures (Kotchoubey et al., 2001; Rockstroh et al., 1993).
Contrarily, when applied to ADHD, negative shifts are encouraged in the hopes that the subjects can retain the skills required to reproduce the general activation and apply it when concentration is required (Strehl et al., 2006; Drechsler et al., 2007). Innovative ways to have children apply the method to their everyday life have been proposed as well. For example, they are given a card on which is displayed an image of the feedback they received while training, and are instructed to try to replicate the self-regulation while looking at the card. Results have been encouraging, and there is more significant validation work currently underway (Riss, 2008).
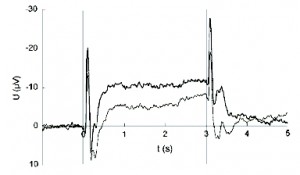
Figure 2. Average CNV during assessment of migraine disorder. Migraine patients appear to produce increased negative amplitudes in the pain-free interval (thick line) compared with healthy controls (thin line). Protocol and method of analysis are similar to the Tubingen approach shown in Figure 1. Taken from Kropp et al, 2002. Note: scale is positive DOWN.
Several other applications exist, including depression (Schneider et al., 2005a), substance abuse (Schneider et al., 2005c) and schizophrenia (Gruzelier et al., 1999; Schneider et al, 2005b), to name a few. A large list of abstracts has been compiled to serve as a recent review of literature regarding self-regulation of SCP (Langley, 2001). Researchers at the University of Kiel have applied this same technique to the assessment and treatment of migraine (Siniatchkin et al., 2000a; Siniatchkin et al., 2000b; Kropp et al., 2002) (Figure 2). As seen in Figure 3, migraine sufferers exhibit increased amplitudes as well as reduced habituation of the Contingent Negative Variation, or CNV – a negative SCP shift in response to preparation (Andrasik and Rime, 2007). During training, subjects are taught to suppress negative SCP shifts to reduce frequency and intensity of migraine attacks. An adaptation of the Tubingen protocol is suggested as a standard for assessment and training of SCP applied specifically to migraine (Kropp, 2000).
Why is it not so straight forward?
As previously mentioned, the DC and low frequency range of the EEG signal are often selectively omitted from recordings simply because of the complexity involved with their inclusion. Several effects – physiological, mechanical, electrical, and chemical – exist to make the recording of EEG with a DC amplifier somewhat of a challenge. Historically, the best way to deal with this has been to filter out the low frequencies. To properly acquire and analyze DC-EEG, the issues involved must be well understood and carefully accounted for, as they have been in almost all of the literature on the topic, at least as experienced by the author of this article. That is not to say that a DC amplifier is not a wonderful tool, only that it comes with great responsibility. Or, more simply put: there is no free lunch!
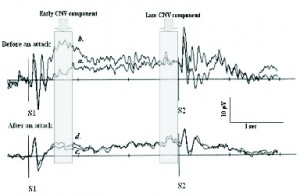
Figure 3. Average CNV in migraine patients, with focus on early and late CNV components. Traces A and B in top pane were recorded the day before an attack, traces C and D were recorded 2 days following. Traces A and C were measured during stress, traces B and D during rest. Trace B during stress and before an attack, a clear increased negative CNV can be seen. Taken from Andrasik and Rime, 2007. Note: scale is positive DOWN.
DC drift
First things first, the DC value of the EEG, or the baseline about which the signal oscillates, actually changes over time. This effect is called DC drift. The rate of this drift is influenced by several factors, the most significant of which is electrode polarization. Electrode polarization is the electro-chemical effect that exists when a metallic electrode is placed in contact with an electrolyte (the conductive gel in this case) and the scalp. A chemical reaction begins during which ions are attracted to the surface of the electrode and charge begins to accumulate. This occurs at each electrode site in different quantities and at different rates, and this discrepancy in charge accumulation creates a voltage that is measured by the system (a.k.a. the battery effect). To make a long story short, the system ends up measuring this additional voltage in parallel with the EEG.
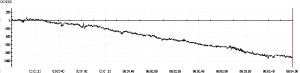
Figure 4. Raw, unfiltered DC-EEG captured with sintered silver/silver/chloride electrodes, shortly after placement. Note the floating baseline (downward in this case) and the very high drift rate of almost 1000 uV in 4 mins (approx. 250 uV / minute). While this drift does decline after some time has elapsed and electrode polarization has reached equilibrium, it is always present in DC-EEG.
The only way to completely remove this effect is to completely remove the low frequencies. When this is not an option, efforts to minimize the effect of DC drift should be made. Also, more important than equipment specifications or technical features, the experimental or clinical paradigm should be such that the effect of DC drift, and all other low frequency artifacts, is inherently ignored by the design of the protocol (more on this later).
To minimize the effect of DC drift, specific amplifier characteristics are required and special electrodes must be used. The amplifier requirements are not so stringent, and are met by most modern commercial DC systems. The special electrodes are made of sintered silver-silver chloride. The term sintered implies a specific manufacturing process, by which the chloride is added to the silver throughout the material and becomes part of the entire electrode, as opposed to comprising a thin sheet on the surface as with regular silver-silver chloride electrodes. A nice comparison of the low frequency performance of most commercially available electrodes was performed by Talgren et al. (2005a), and the results are straight forward: only this type of electrode will do for low frequency recordings. It offers the lowest electrode polarization (and hence contribution to DC drift), the least low frequency noise and the best long-term stability. Several chloride-based conductive pastes are recommended as well (among these are name brands EC2, Ten20, and Electro-Gel).
The sintered Ag/AgCl electrodes themselves are somewhat delicate. They are absorbent and should not be left in contact with any substance for very long, lest they absorb foreign ions and then performance becomes degraded. They should be cleaned immediately after use and never left with any paste affixed that can dry and harden. Ideally they should be rinsed with distilled water and should be left to hang dry. It is also sometimes recommended that before use, soaking them in a saline solution for up to an hour can help reduce polarization and decrease settling time once they are actually applied.
In addition to using these electrodes and pastes, careful preparation and placement techniques should be employed to ensure reliability, lower electrode impedance, and avoid artifacts related to the skin-electrode interface as much as possible.
Skin-electrode interface
Two important requirements for DC-EEG were described in context of DC drift (a stable system with good amplifier specifications, and high quality non-polarizing electrodes with a chloride-based conductive paste). The third important requirement for DC-EEG involves the skin-electrode interface, which is determined by how well the skin is prepared and the electrodes are applied.
Integrity of the skin-electrode interface is crucial to minimizing artifacts due to electrode movement and transdermal potentials (specifically galvanic skin response, or GSR). Tallgren (2005b, 2006) warns that the only way to avoid electrode movement artifacts is to fix the electrode with collodion (a pyroxilin-based surgical adhesive often used to fix surface EEG electrodes for long term recording) or other method (proprietary methods are described) and to ensure a constant amount of paste is always used. Bauer et al. (1989) recommend that the electrode gel be properly evacuated using a vacuum pump to avoid air bubbles in the gel.
Tallgren (2005b, 2006) also warns that the only way to avoid GSR artifact, and to reliably record slow cortical potentials with a DC amplifier is to short circuit the skin, meaning it must be punctured. While this would seem extreme, it is repeatedly mentioned as a critical requirement in DC-EEG. Recent studies by Hennighausen et al. (1993), Bauer et al. (1989), Voipio et al. (2003) and Tallgren (2005b) confirm previous studies by Picton and Hillyard (1972) and Cowen (1974) in which continuous, unpredictable DC drifts and often a profound contamination by GSR were measured when skin was left intact.
Other important effects
Eye movement is another significant source of artifact in DC-EEG recordings. Sometimes confused as ocular EMG, the signal produced by the eye is in fact due to the electrical properties of the eye itself. The eye acts as an electrical dipole and produces a signal that is captured by the EEG amplifier. Eye blinks are well-known contaminants in neurofeedback, but eye movement and even eye position are important in DC-EEG as they contain considerable low frequency content. Eye position is in fact a DC signal itself, and it influences the baseline of the EEG signal; upward gazes will shift the baseline upwards, and downward gazes downwards. In addition to acting as an artifact, in SCP protocols eye movements can actually influence the feedback by mimicking the required positive and negative shifts in the SCP (voluntarily or otherwise).
Respiration causes another, lesser-known physiological artifact in DC-EEG. Voipio et al. (2003) make a strong case for a non-neuronal generator of DC shifts, manifested as negative shifts linked to hyperventilation and positive shifts linked to hypoventilation, both directly caused by changes in partial pressure of carbon dioxide (PCO2) in the brain. Speckmann and Elger (2005) also present a clear positive shift during hypercapnia (increased CO2).
Finally, the influence of inter-subject variability which always helps to keep things interesting. According to Kotchoubey et al (2000), “subjects differ greatly in their ability to learn” and “independently of this, humans differ substantially in their overall tendency to produce positive or negative shifts, regardless of the task.” That said, a quote from Hinterberger et al. (2003) is interesting:
Success in self-regulation training depends not only on the correct selection of technical parameters, but also on the patient’s psychological and physical state, motivation, social context, and the trainer-patient-relationship. Recent data demonstrated that self-regulation and communication skills of severely paralyzed patients could be predicted from the results of the initial training period: Patients who later acquired the self-regulation skill well enough to communicate (i.e., > 75% correct responses), had already showed a high performance (>80%) in the first 30 training sessions (about three training days [at 7-10 sessions per day, 4-8 minutes per session]) (Neumann and Birbaumer, 2003). Attentional capacities and motivational factors might be responsible for these performance differences between patients.
Siniatchkin et al. (2000b) also confirm previous reports that children and adults can successfully learn self-regulation of SCP within 2 sessions.
What has been done?
It would appear nearly impossible to work with DC-EEG with all these sources of artifact and ambiguity. An obvious question arises: How has self-regulation training of SCP been achieved if simply measuring the signal is so complicated? Thankfully there has been significant innovative work that has shown solid results through practical methodology, with the a priori intention of influencing clinical practice to follow suit.
AC vs. DC
The suggestion has been made that DC-EEG is the only way to record slow potentials (Voipio et al., 2003; Tallgren, 2006). Even more benefits are appreciated when no filters whatsoever are applied, and the more apt term full-band EEG, or fbEEG, is adopted (Vanhatalo et al., 2005). These are certainly valid arguments, and the authors make a strong theoretical case as applied to several areas of EEG analysis. For real-time neurofeedback however, DC-EEG is simply too impractical for use in a clinical setting. The authors agree, as mentioned above, that an absolute requirement, at the very least, is to puncture the skin. Co-author Pekka Tallgren, in his own comprehensive treatise on clinical DC-EEG (Tallgren, 2006), concludes after having tested several different methods to short circuit the skin, that there is no simple, quick, practical solution and that this remains a significant problem in the use of DC-EEG for clinical work.
In fact, most often DC amplifiers have not been used in the research of low frequency EEG because the complexity simply outweighs the benefits. All of the research quoted in this article, as well as that of many other studies, was mostly carried out using low frequency AC amplifiers (amplifiers that omit the DC component). Birbaumer’s original Thought Translation Device (Birbaumer et al, 2003) and much of the work at Tubingen was implemented with a PSYLAB EEG8 amplifier from Contact Precision Instruments, which not surprisingly has a low frequency cutoff of 0.01 Hz.
That said, a DC amplifier can certainly be used, and would indeed record a theoretically pure SCP, but until more research is done and new discoveries are made, the DC-EEG issues outlined above cannot be ignored. The cost involved with using an AC amplifier is that a small portion of the SCP signal is lost. Also, the dynamics of the AC amplifier are such that recovery from movement artifacts require quite a long time. Thus, failing to identify these artifacts renders the recording useless, and avoiding or removing them becomes mandatory.
The benefit is a significant reduction in DC drift, although several of the same exogenous artifacts still exert influence albeit to a lesser degree. In essence, the extent of the trade-off depends on the cutoff of the AC amplifier. The lower it is, the more DC effects are included; the higher it is, the less pure SCP is recorded. The usual operating point is such that some low frequency effects are tolerated and an acceptable (although unknown) amount of SCP is filtered.
Standard Protocols based on the Tubingen Research
Electrode placement is at the vertex (‘Cz’ in the International 10-20 system) referenced to linked mastoids. To differentiate the SCP from DC drift and other low frequency artifacts, the mean SCP amplitude is computed relative to a trailing baseline and the clinical protocols are designed to isolate the positive and negative shifts from the background EEG. The general idea is that self-regulation via visual or auditory information is coordinated with an “effector,” either motor (push button response) or non-motor (imagery and thinking) that explicitly elicits SCP output (Kotchoubey et al., 2000).
This is achieved using oddball paradigms and multi-trial averaging, such that repeated efforts to move the SCP in one direction or another are averaged separately. This ensures that what is trained and measured is in fact the self-induced shifts that are required as immediate responses to the stimuli pairs (target and non-target, for example; or positive and passive, negative and passive, or positive and negative). It also allows measurement of the respective SCPs, and hence the ability to quantify the progress of the self-regulation training by reporting an actual amplitude reading for a given session.
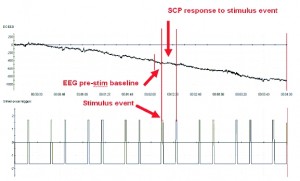
Figure 5. DC-EEG shown with timeline of stimuli event triggers (indicating when the stimuli were presented). The pre-stim baseline is defined as directly before the stimulus, and the response SCP is immediately after. Before averaging, the baseline is removed from the SCP response such that what is captured is the explicit change in slow EEG output in response to the self-regulation cue, as mentioned in Kotchoubey et al., 2000.
An important point is that amplitude of each SCP is measured relative to the section of EEG immediately prior to the generation of the shift (Figure 5), such that the immediate change in amplitude of each trial is averaged during the period in which SCP shifts persist. This is the final prerequisite that, when combined with the other protocol parameters, allows the differentiation of the SCP and DC drift and other low frequency artifacts.
To maximize learning, positive and negative shifts are randomly distributed. Trials also exist both with and without feedback. Feedback is given when the individual SCP shift produced is large enough to reach a threshold. The trials without feedback are called transfer trials as they are designed to transfer the ability to produce shifts in real life situations, when no feedback exists.
The number of sessions varies, but in general the strategy includes groups of sessions separated by periods of non-training. For example, one of Dr. Strehl’s ADHD protocols includes thirty 1-hour sessions, in three groups of ten, with each group lasting two weeks (five days per week) and separated by a 4-6 week break (Strehl et al., 2006).
To eliminate the influence of EOG and the CO2 effect
Eye movements should be discouraged as much as possible. The gaze of the subject should be fixed and the subject should be instructed only to blink between trials (to avoid eye blinks being included in the trial averages).
In a slightly more advanced approach, EOG can be measured simultaneously and each or any combination of the following can be done: feedback can be interrupted when EOG activity exists, the EEG recording can be marked to indicate presence of EOG, and the EOG can be removed offline.
The Birbaumer group in most cases performs the subtraction online to ensure that feedback is not given for eye movements. This is implemented using a direction-independent method such that eye movement interrupts feedback but does contribute to it (by moving in the opposite direction, for example) (see Kotchoubey et al., 2000). Offline correction is also performed before analysis of the averages. This is a very useful technique that would seem to apply very well to general artifact removal in any type of biofeedback protocol. The online method does not necessarily have to be perfect, as long as processing times are fast and feedback is not influenced, and the associated offline method can require more processing power to remove artifacts accurately for the analysis of the data.
Respiration can also be measured to ensure that breathing remains calm and rhythmic (e.g. 4-6 breaths per minute). At the very least, breathing can be monitored visually without necessarily recording respiration, and the practicing clinician can respond to irregular breathing appropriately.
Conclusion
All of the technical and scientific challenges presented in this article are by no means meant to frighten or deter the reader from further exploring DC-EEG and self-regulation of SCPs. On the contrary; they are presented to inform so that as the new techniques are adopted, the likelihood of positive clinical outcomes is maximized. An analogous discussion could be about driving a car, in which the concepts of a gas pedal and brakes, a clutch and stick shift, a steering wheel, road conditions, traffic signs, pedestrians and speeding tickets may seem frightening and may even be strong deterrents. Most of us drive a car every day without considering these things to be overwhelming challenges, yet we are intimately aware of each and every one of them every time we get behind the wheel.
Clearly all the successful researchers who obtained results both experimentally and clinically with SCPs were well aware of these DC-EEG issues, and they managed, through this knowledge and with much creativity and determination, to achieve applicable results. As a clinician, thankfully you need not reinvent the wheel. That is the point of relying on research: you stand, so to speak, on the shoulders of giants. At the same time, clinical work is the loop that closes the circle, and research cannot truly progress without a venue for the applications it suggests. True progress is gained through collaboration, and only a consistent effort can continue to drive the field.
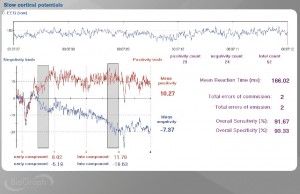
Figure 6. BioGraph Infiniti screen showing voluntary SCP production of positivity (red) and negativity (blue). Also displayed are mean values for early and late SCP components. Mean total SCP values are shown along the right. Reaction time statistics are also displayed here, and are optionally available in parallel with SCP measurements as they reflect attention and focus (low errors) and motor readiness (low mean reaction times). Note: scale is positive UP.
If you would like to know more about DC-EEG, SCP and assessment and training techniques using Thought Technology’s new line of SCP equipment for the Infiniti platform (Figure 6.), please contact Marc Saab at Thought Technology.
Mark Saab
Thought Technology
Editorial Comment From CFisher:
Those who are not familiar with NeuroConnections can now better understand of the quality of their articles. NeuroConnections is a free benefit of membership from the International Society for Neurofeedback and Research (ISNR) and available only in printed format. I encourage all professionals who specialize in neurofeedback, biofeedback or QEEG to join ISNR to receive NeuroConnections and other member benefits. The above article was reprinted from NeuroConnections (January, 2009) with permission from ISNR.
References:
Andrasik F, Rime C. Can behavioural therapy influence neuromodulation? Neurol. Sci.; 28: S124-S129, 2007.
Bauer H, Korunka C, Leodolter M. Technical requirements for high-quality scalp DC recordings. Electroencephalography and Clinical Neurophysiology; 72: 545-547, 1989.
Birbaumer N, Elbert T, Canavan GM, Rockstroh B. Slow potentials of the cerebral cortex and behaviour. Physiol. Rev.; 70: 1-41, 1990.
Birbaumer N, Kübler A, Ghanayim N, Hinterberger T, Perelmouter J, Kaiser J, Iversen I, Kotchoubey B, Neumann N, Flor H. The thought translation device (TTD) for completely paralyzed patients. IEEE Trans. Rehab. Eng.; 8: 190-193, 2000.
Birbaumer N, Hinterberger T, Kübler A, Neumann N. The Thought-Translation Device (TTD): Neurobehavioral Mechanisms and Clinical Outcome. IEEE Trans. on Neural Sys. and Rehab Eng.; 11(2), 2003.
Cowen MA. The brain as generator of transcephalically measured direct current potentials. Psychophysiol.; 11:321-35. 1974
Drechsler R, Straub M, Doenhart M, Heinrich H, Steinhausen HC, Brandeis D. Controlled evaluation of a neurofeedback training of slow cortical potentials in children with ADHD. Behavioral and Brain Functions; 3(35), 2007
Geary J. The Body Electric: An anatomy of the new bionic senses. Rutgers University Press. Piscataway, NJ. 2002.
Gruzelier JH, Hardman E, Wild J, Zaman R. Learned control of slow potential interhemispheric asymmetry in schizophrenia. International Journal of Psychophysiology; 34: 341-348, 1999.
Hennighausen E, Heil M, Rosler F. A correction method for DC drift artifacts. Electroencephalography and Clinical Neurophysiology; 86: 199-204, 1993.
Hinterberger T, Houtkooper JM, Kotchoubey B. Effects of feedback control on slow cortical potentials and random events. The Parapsychological Association Convention; Vienna, Austria. 2004
Hinterberger T, Schmidt S, Neumann N, Mellinger J, Blankertz B, Curio G, Birbaumer N. Brain-computer communication and slow cortical potentials. TBME-00279-2003, 2003.
Kotchoubey B, Haisst S, Daum I, Schugens M, Birbaumer N. Learning and self-regulation of SCP in older adults. Experimental Aging Research; 26: 15-35, 2000.
Kotchoubey B, Strehl U, Uhlmann C, Holzapfel S, König M, Fröscher W, et al. Modification of slow cortical potentials in patients with refractory epilepsy: A controlled outcome study. Epilepsia; 42: 406-416, 2001.
Kropp P, Kiewitt A, Göbel H, Vetter P, Gerber Wd. Reliability and stability of contingent negative variation. Applied Psychophysiology and Biofeedback; 25 (1): 13-32, 2000.
Kropp P, Siniatchkin M, Gerber WD. On the pathophysiology of migraine – links for “empirically based treatment” with neurofeedback. Applied Psychophysiology and Biofeedback; 27 (3): 203-213, 2002.
Kübler A, Kotchoubey B, Hinterberger T, Ghanayim N, Perelmouter J, Schauer M, Fritsch C, Taub E, Birbaumer N. The thought translation device: a neurophysiological approach to communication in total motor paralysis. Exp. Brain Res.; 124: 223-232, 1999.
Kübler A, Kotchoubey B, Kaiser J, Wolpaw J, Birbaumer N. Brain-computer communication: unlocking the locked-in. Psych. Bull.; 127(3): 358-375, 2001.
Langley Z. Slow cortical potentials: neurobehavioral management of epileptic seizures – An interview with Dr Ute Strehl. 2001. Personal website of Professor Zoe L. Langley, Indiana University. Last accessed Dec 2008: http://www.indiana.edu/~pietsch/zoestrehl.html
Langley Z. Slow cortical potentials: A survey of the recent literature. 2008. Personal website of Professor Zoe L. Langley, Indiana University. Last accessed Dec 2008: http://www.indiana.edu/~pietsch/scp.html
Neumann N, Birbaumer N. Predictors of successful self-control during brain-computer communication. Journal of Neurology, Neurosurgery, & Psychiatry; 74(8): 1117-1121, 2003.
Picton TW, Hillyard SA. Cephalic skin potentials in electroencephalography. Electroencephalography and Clinical Neurophysiology; 33(4): 419-424, 1972.
Riss R, Strehl U. Slow Cortical Potentials in the Treatment of ADHD – An interview with Ute Strehl. NeuroConnctions newsletter. ISNR and AAPB, October 2008.
Rockstroh B, Elbert T, Birbaumer N, Wolf P, Düchting-Röth A, Reker M, et al.. Cortical self-regulation in patients with epilepsies. Epilepsy Research; 14: 63-72, 1993.
Schneider F, Heimann H, Mattes R, Lutzenberger W, Birbaumer N. Self-regulation of slow cortical potentials in psychiatric patients: depression. Applied Psychophysiology and Biofeedback; 17 (3): 203-214, 2005.
Schneider F, Rockstroh B, Heimann H, Lutzenberger W, Mattes R, Elbert T, Birbaumer N, Bartels M. Self-regulation of slow cortical potentials in psychiatric patients: schizophrenia. Applied Psychophysiology and Biofeedback; 17 (4): 277-292, 2005.
Schneider F, Elbert T, Heimann H, Welker A, Stetter F, Mattes R, Birbaumer N, Mann K. Self-regulation of slow cortical potentials in psychiatric patients: alcohol dependency. Applied Psychophysiology and Biofeedback; 18 (1): 23-32, 2005.
Siniatchkin M, Hierundar A, Kropp P, Kuhnert R, Gerber WD, Stephani U. Self-regulation of slow cortical potentials in children with Migraine: an exploratory study. Applied Psychophysiology and Biofeedback; 25 (1): 13-32, 2000.
Siniatchkin M, Kropp P, Gerber WD. Neurofeedback – The significance of reinforcement and the search for an appropriate strategy for the success of self-regulation. Applied Psychophysiology and Biofeedback; 25 (3): 167-175. 2000.
Speckmann EJ, Elger CE. Introduction to the Neurophysiological Basis of the EEG and DC Potentials, Chapter 2 in Neidermeyer E, Lopes da Silva F. Electroencephalography: Basic principles, clinical applications and related fields. 5th edition. Lippincott Williams & Wilkins. New York: 2005.
Strehl U, Leins U, Goth G, Klinger C, Hinterberger T, Birbaumer N. Self-regulation of Slow Cortical Potentials: A New Treatment for Children With Attention-Deficit/Hyperactivity Disorder. Pediatrics; 118: 1530-1540, 2006.
Talgren P, Vanhatalo S, Kaila K, Voipio J. Evaluation of commercially available electrodes and gels for recording of slow EEG potentials. Clinical Neurophysiology; 116: 799-806, 2005.
Tallgren P. DC-stable electrode-skin interface for human EEG recordings. Helsinki University of Technology, Applied Electronics Laboratory, Series E: Electronic Publications, E5: 3-12, 2005.
Talgren P. DC-EEG for routine clinical use: methods and clinical impact. Doctoral dissertation – Applied Electronics Lab., Helsinki University of Technology – Helsinki. ISBN 951-22-6955-4. 2006.
Vanhatalo S, Voipio J, Kaila K. Full-band EEG (FbEEG): an emerging standard in electroencephalography. Clinical Neurophysiology; 116: 1-8, 2005.
Voipio J, Tallgren P, Heinonen E, Vanhatalo S, Kaila K. Millivolt-Scale DC Shifts in the Human Scalp EEG: Evidence for a Nonneuronal Generator. Journal of Neurophysiology; 89: 2208-2214, 2003.
Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin. Neurophysiol.; 113(6): 767-791, 2002.
No comments yet.